Glossary
How much does desalination cost?
Desalination solutions are relevant in contexts of limited accessibility to a fresh water source, because the process, even optimized, remains more complex than a treatment of salt free water.
Depending on the volume produced per day, the raw water quality (salt concentration and other components to be treated) and the energy consumption of the desalination plant, the water production costs range between 0.5 and 3€ per m3.
Why using solar energy to power desalination plants?
- Solar energy is the most cost competitive energy source, though a fluctuating source,
- Solar energy generates among the lowest greenhouse gas emissions of all energy sources
- Solar energy is largely available is water scarce regions of the world
What is the difference between sea water and brackish water?
Sea water: it is the salt water of the earth's seas and oceans. it is said to be "salty" because it contains dissolved substances, salts, made up of ions, mainly halide ions such as the chloride ion and alkaline ions such as the sodium ion. there are 30 to 40 grams of dissolved salts in 1 kg of sea water.
Waters may be made brackish by two processes:
- saline infiltration of nearby seawater into permeable subsoil.
- infiltration of (fresh) rainwater into subsoil that is geologically rich in minerals, including salts, and potentially other minerals. The chemical composition of brackish water may vary greatly as a result.
How is salinity measured?
- by measuring weight using the gram, i.e. 35g of salts per liter of seawater on average several units can be used: mg/l = g/m3 = ppm
- by measuring the specific conductivity, i.e. the total content of dissolved salts. it is measured in µm / cm at 25 degrees Celsius.
What is turbidity ?
Turbidity : this refers to the content of suspended particles in the water that cause it to become turbid. These suspended particles may be organic or not. Turbidity is measured in NTU (nephelometric turbidity unit).
- reverse osmosis
- distillation
- electrodialysis
- ion exchange
Reverse osmosis : It is the process used today for 95% of new desalination plants, but also, at low pressure, for the potabilization of fresh water. Osmosis is a natural phenomenon of equilibrium of forces between two liquids, and which allows a low concentrated liquid to pass through a membrane to dilute a more concentrated medium. This principle is for instance implemented by the cells of the human body. These membranes are said to be semi-permeable, allowing only water molecules to pass through.
How does reverse osmosis work ?
Let's say that one of the media is highly concentrated in salt (such as sea water), the less concentrated medium (fresh water) will migrate to the more salty medium.
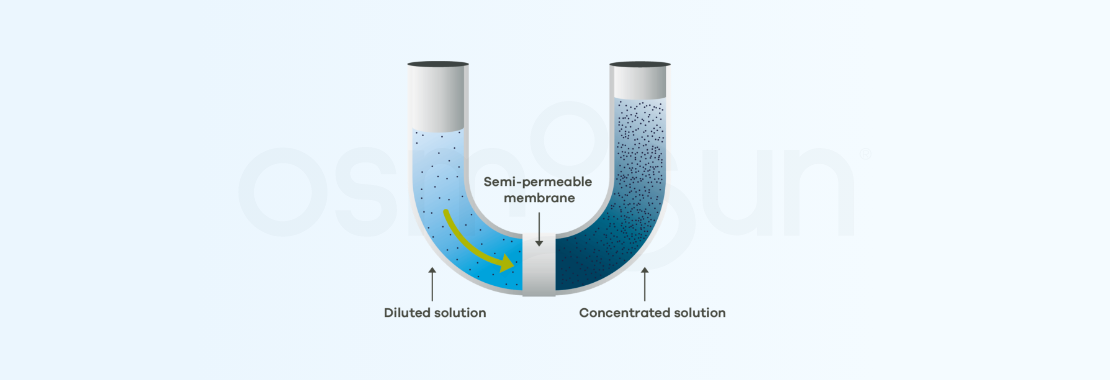
It can be noted that a difference in level between the media will be created as a result of the displacement of water. At equilibrium this height will result in a pressure called osmotic pressure.
The process can be reversed. To do so, a pressure is applied in the highly concentrated medium, which must be greater than the osmotic pressure (in practice, twice as much). pure water is therefore recovered on the other side of the membrane. This is reverse osmosis.
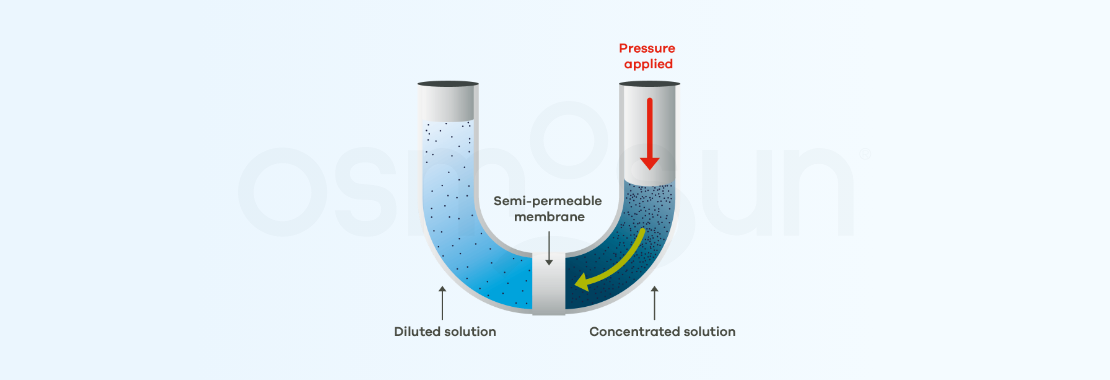
In the previous diagram, salts accumulate on the membrane as desalination proceeds. In order to limit membrane fouling, industrial systems use reverse osmosis in tangential filtration.
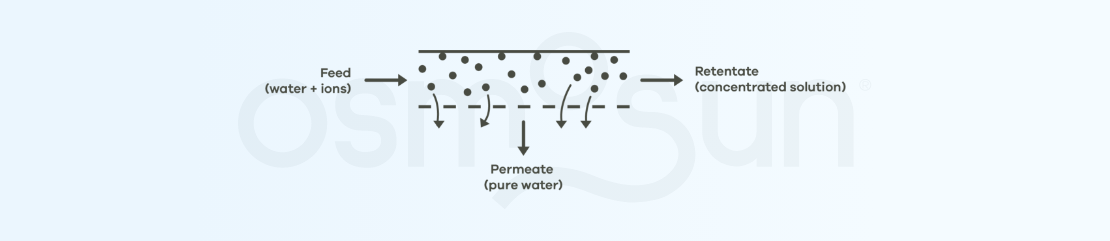
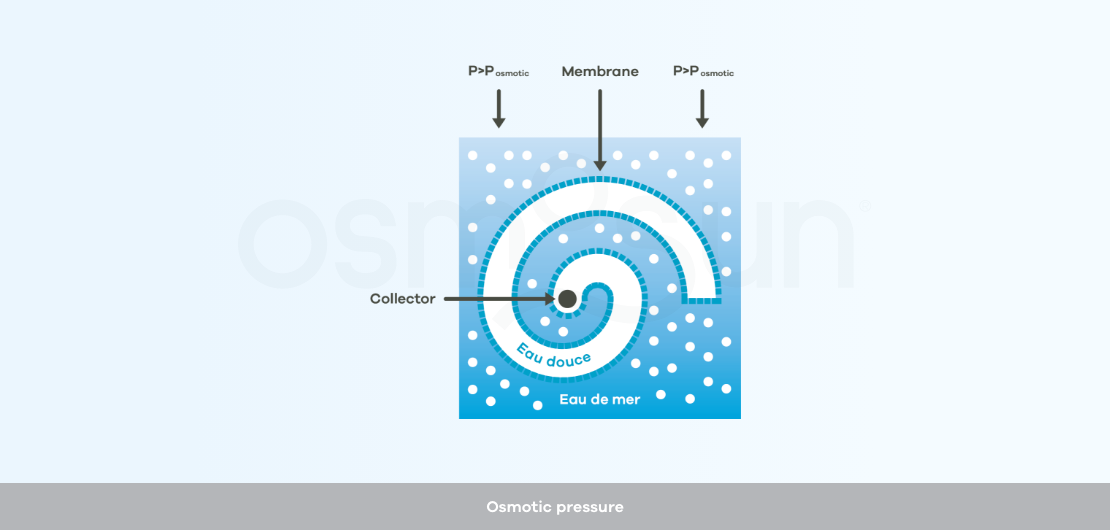
What do reverse osmosis membranes look like?
Reverse osmosis membranes : refers to semi-permeable membranes as described above, allowing water treatment by reverse osmosis. The manufacturing of industrial reverse osmosis membranes has been optimized, the membranes are usually spiral wound.
Part of the feed flow (raw water) passes through the membrane: this is the permeate, the fresh water we want to obtain. The part that does not pass through the membrane is the concentrate.
- the quality of the fresh water produced: reverse osmosis not only retains salts, it also retains organic matter, viruses and bacteria, pesticides and herbicides. The water produced by reverse osmosis is therefore drinkable.
- the ease of installation and the compactness of the systems, whether for seawater desalination (swro) or brackish water desalination (bwro)
- the low specific energy consumption, i.e. in relation to the m3 produced, around 3.5 kwh/m3 for seawater.

OSMOSUN® specificity:
For seawater at 35 g/l 25°c, the OSMOSUN® process uses 2,2 kwh/m3, excluding raw water pumping, the consumption of which depends on the installation on site.
What does the recovery mean?
Recovery: This is an important concept in reverse osmosis projects. It represents the difference between the permeate flow produced (fresh water) and the raw water feed flow.
This recovery rate is equal to the volume of fresh water produced divided by the volume of raw water pumped:
- for seawater desalination, it is generally produced between 30 and 50 l of drinking water from 100 l of raw water, equivalent to a recovery between 30 and 50%
- for brackish water desalination, the recovery is between 65 and 80%.
Permeate: this is the technical term of presenting drinking water in a reverse osmosis process, i.e. the liquid that has passed through the membrane of a chemical separation process (which can be reverse osmosis or ultrafiltration).
How does the energy recovery device work?
Energy recovery : It is used in seawater desalination to achieve low energy consumption. These systems use the pressure of the concentrate (which can be as high as 80 bar) to pressurize the seawater entering the membranes. The most efficient technology, and the most widespread on the market, is the pressure exchanger.
For a dynamic explanation of the pressure exchanger, see the video provided by Danfoss for its isave system: www.youtube.com/watch?v=j81mctv7tuw. There are other systems such as: ERI (energy recovery inc., isobaric pressure exchanger), DWEER (dual work exchanger energy recovery from flowserve) for example.
Today, the reverse osmosis process is technologically mature. However, it still requires energy, usually fossil fuels (generator, or electrical networks often using fossil fuels). However, it is possible to power reverse osmosis units with renewable energy, the most advanced systems are those of solar desalination.

OSMOSUN® specificity:
the patented OSMOSUN® reverse osmosis process allows a solar photovoltaic power supply without batteries, thus a variable power operation according to the sun, despite the pressure levels required for desalination (up to 80bars), and the importance of not subjecting the membranes to any pressure fluctuation higher than 0.7 bar/second
What are the different types of fouling of reverse osmosis membranes?
fouling: refers to an accumulation of particles in the reverse osmosis membranes that affects their production performance (lower flow rate, higher pressure, higher permeate salinity), highlighting the importance of pre-treatment upstream of an efficient reverse osmosis process.
What is biofouling?
Biofouling: in the reverse osmosis treatment, it is the formation of a disturbing layer of living beings on the surface of the membrane in permanent or frequent contact with the sea water. This is facilitated when the water to be treated that is rich in organic matter. An effective pre-treatment avoids this phenomenon.
What is scaling?
Scaling: it is the clogging of reverse osmosis membranes by precipitates. An injection of anti-scaling agent at a very low dose, a few parts per million (ppm) will prevent this phenomenon in the membranes.
Why is an antiscalant used for reverse osmosis desalination ?
Antiscalant: it is a liquid injected upstream of the reverse osmosis process, as a pre-treatment, in order to prevent the formation of scale on the surface of the membranes, via the precipitation of mineral salts. This inhibition of scale makes it possible to maintain the performance of reverse osmosis membranes.
Why performing a chemical cleaning of reverse osmosis membranes?
Chemical cleaning: in the maintenance of reverse osmosis equipment, it is a method to remove unwanted contaminants from the membranes. Chemical cleaning is carried out punctually -every 2 to 6 months- to regenerate the performance of reverse osmosis membranes, through the successive circulation in the membranes of acid and basic solutions.
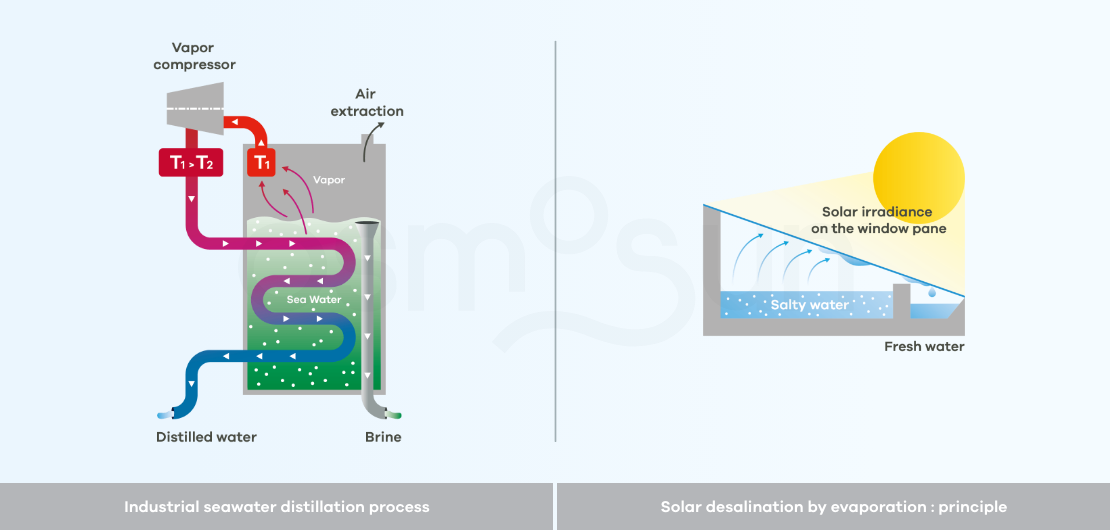
How does desalination by distillation work?
Distillation: consists of heating salt water to evaporate the water, then condensing it, to obtain pure water. It is a very energy consuming process. There are industrial processes but also low-tech processes with low production capacity (about 5 l/day/m2).
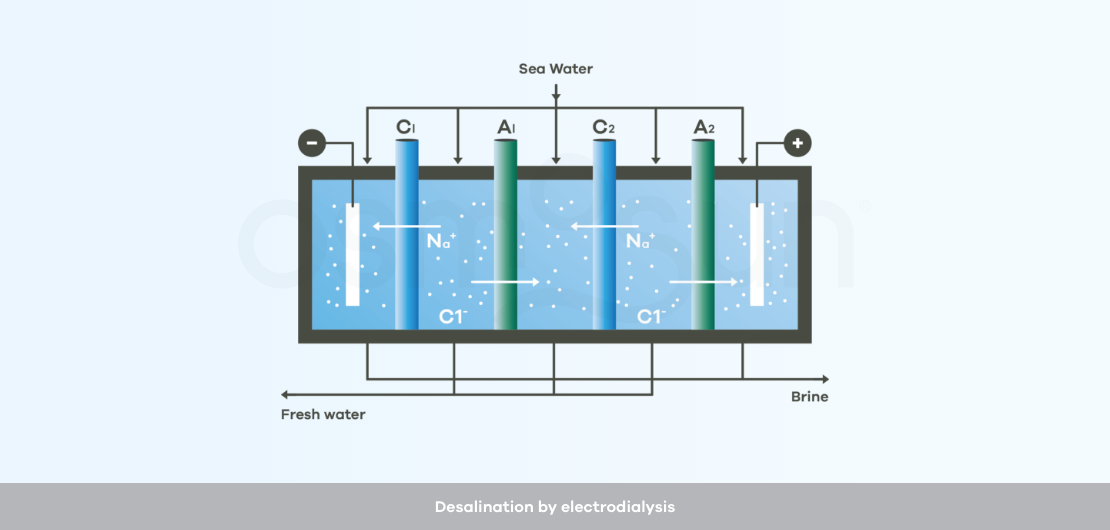
How does desalination by electrodialysis work?
Electrodialysis : uses electrodes and membranes that are permeable to positive ions and others that are permeable to negative ions. The saltier the water to be treated, the higher the energy consumption.
Electrodialysis only desalts the water, it does not make it drinkable, i.e. pathogens are not eliminated. One or more additional treatment stages are thus necessary before human consumption.
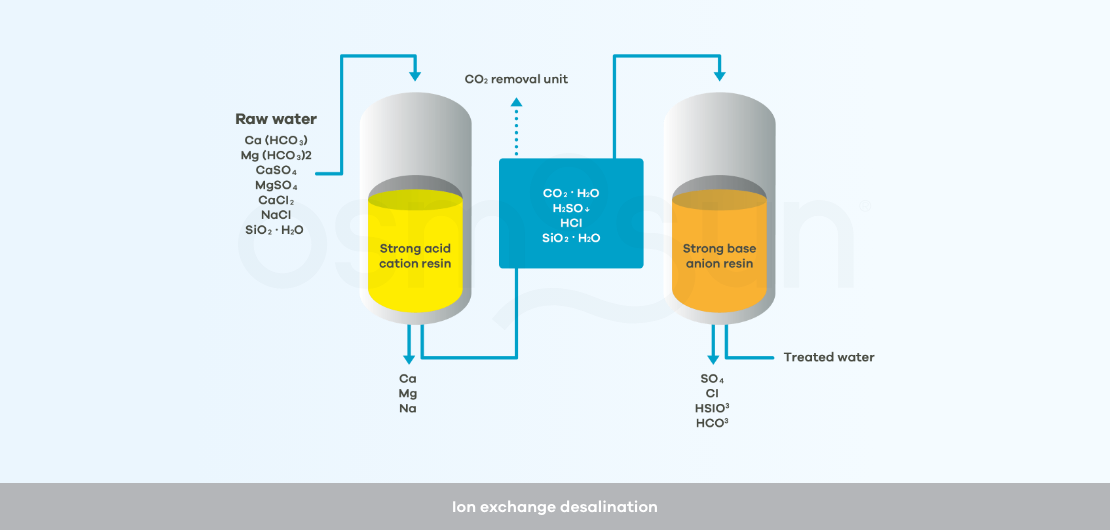
How does ion exchange desalination work?
Ion exchange : a solution reserved for demineralizing water with a low salt concentration, < 1 g/l. It consists of circulating the raw water through successive resins to eliminate the salts.
Reverse osmosis desalination project: such a project gathers different components, which may be broken down as follows:
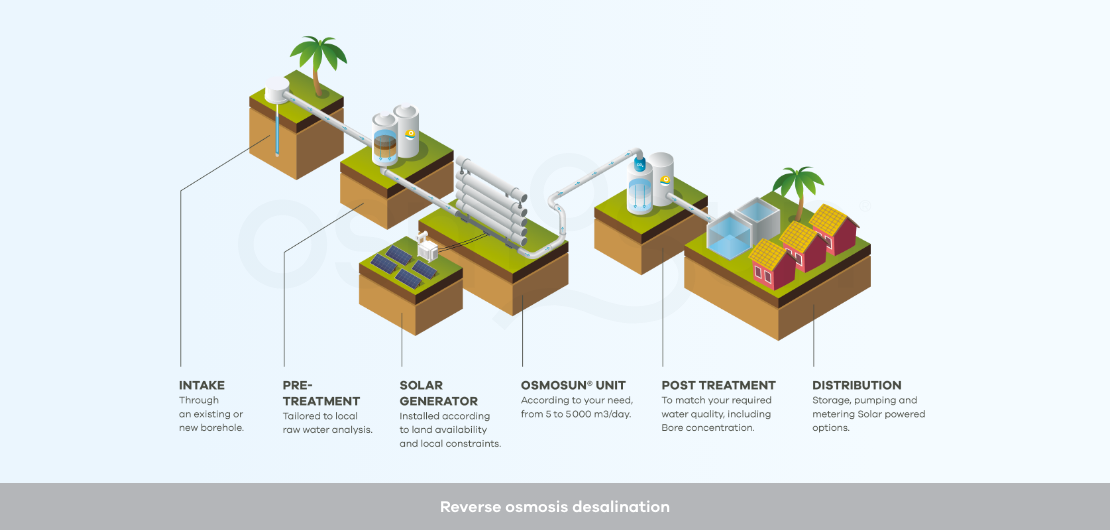
Raw water intake: it is crucial to offer a stable water quality over the seasons and potential bad weather conditions to the reverse osmosis membrane process. Rather than taking water directly from the sea, subject to the tides and rich in suspended solids, OSMOSUN® recommends the use of coastal drilling to benefit from the natural filtration of the soil, if the local geology allows.
Pre-treatment: they are necessary upstream of reverse osmosis, because this process requires water with almost no turbidity, no particles larger than 5 microns, as little organic matter as possible, and as few ions as possible that could generate precipitates. The aim is to avoid fouling (clogging by particles), scaling (clogging by precipitates) and biofouling (clogging by organic matter).
There are different physico-chemical, biological or membrane processes for water pre-treatment: coagulation, flocculation, decantation, sand filtration, oxidation, activated carbon adsorption, microfiltration, ultrafiltration, deironing, demanganisation, antiscalant injection... The range of technologies depends on the local context and the water analysis OSMOSUN® may design and supply the solution adapted to each need.

OSMOSUN® specificity:
Some pre-treatment systems use a significant number of chemicals. In projects where OSMOSUN® is responsible for the entire water treatment chain, we study on a case-by-case basis the possibility of eliminating or reducing the quantities used.
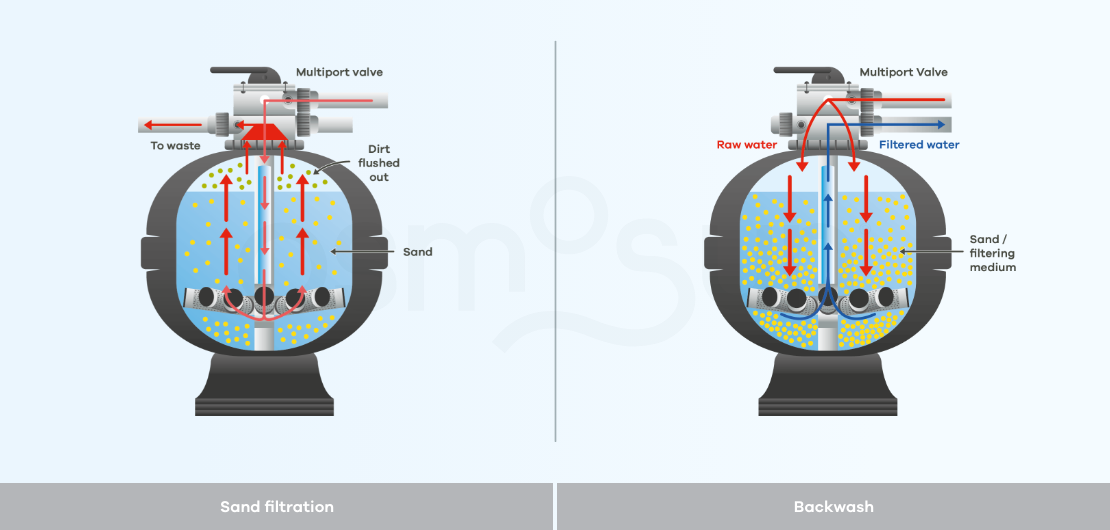
How does a sand filter work?
A sand or multimedia filter is a filtration technique called physico-chemical, which consists of a tank filled with a filtering medium, which can be sand, anthracite, etc.
The operation of this filtration is very simple: raw water loaded with impurities is pumped into the tank where it is filtered by the sand which retains the impurities. The cut-off point of the sand filter is between 30 and 40 microns.
Backwash, is a maintenance process by which a filter is cleaned. This cleaning can be done either by reversing the flow of water through the filters, by agitating the filter beds with air or other mechanical means, or by directing jets of water or air onto the screens to clean them. This should be done regularly to maintain the performance of the filters.
What is the use of coagulation and flocculation processes?
Coagulation and flocculation: these are pre-treatment processes that facilitate the elimination of suspended matter by gathering it in the form of floc, which is then separated by decantation, flotation and/or filtration systems.
Floc : refers to the agglomeration of particles by different water treatment processes: flocculation / coagulation.
How to treat iron in water?
Iron : it is an element widely present in soils, and which is naturally found in the water to be treated. A specific treatment is required due to the inadequacy of most traditional physico-chemical pre-treatment systems to get rid of it. The solution commonly used consists of biological oxidation of iron by oxygen, in order to aggregate the different iron ions to create flocs, which can then be eliminated by filtration systems on filtering media such as sand.
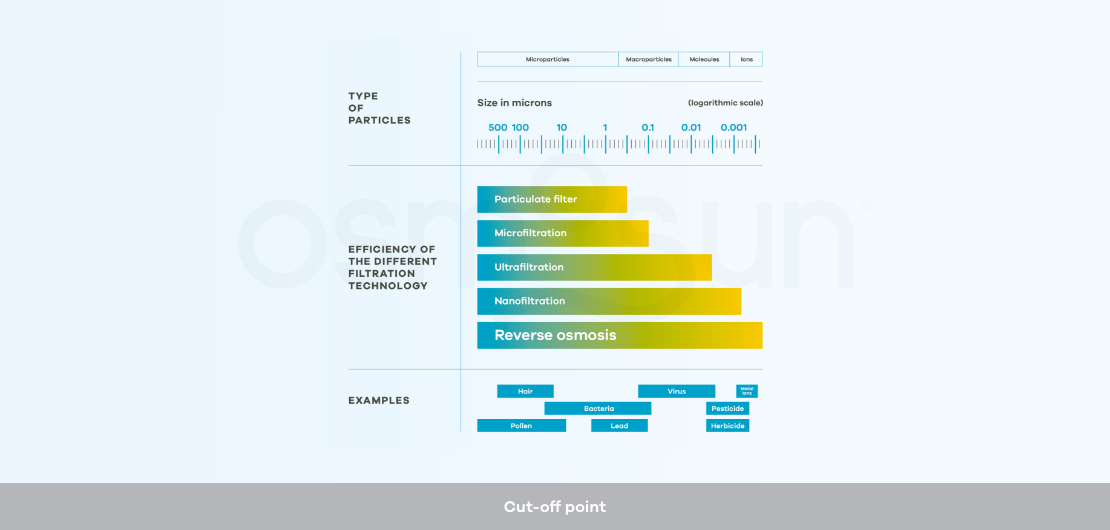
What is ultra-filtration?
Ultra-filtration: is a membrane separation method with a lower cut-off point than reverse osmosis. It is used as a pre-treatment in desalination processes by reverse osmosis, or alone as a solution for the potabilization of non-saline water.
What is the cut-off point?
Cut-off: refers to the mass of the smallest molecule or ion that is stopped by an ultrafiltration, nanofiltration or reverse osmosis membrane under standard conditions.
We find the following systems:
- Remineralization: interesting in the case of injection of the reverse osmosis permeate in a public drinking water network. This process consists in bringing the water to a calco-carbonic equilibrium, as well as to a ph close to neutrality (neutral ph = 7). Different techniques can be used, together or separately: calcite filters, lime milk injection, sodium bicarbonate, calcium chloride, magnesium chloride, etc.
- Disinfection : to ensure the absence of pathogens in the water network or in the reservoirs, ultraviolet rays (UV) can be used, but this does not allow the water to be stored (no residual effect) In order to have potable water over time, it is preferable to inject chlorine, often in the form of sodium hypochlorite.

OSMOSUN® specificity:
OSMOSUN® has developed an autonomous solar chlorination solution that allows the production of this agent locally, with a direct and controlled injection of the solution into the drinking water produced, thus ensuring the non-proliferation of pathogenic organisms.
Can the desalination unit be containerized?
The reverse osmosis desalination unit, as well as the associated pre- and post-treatment, can be installed in a container to facilitate deployment, although this is not mandatory. The installation of a RO unit in a tropical climate and in a marine environment requires certain precautions, in order to prevent corrosion of the steel container or insulation associated with ventilation or air conditioning of the container to limit the preservation temperature of the equipment.
What can be done to avoid corrosion on a desalination project?
Corrosion : refers to the alteration of a material by chemical reaction with an oxidant: most metals suffer from corrosion, forming rust, when exposed to salt and oxygen. This phenomenon is anticipated in desalination processes by using stainless steels -inox, which is an alloy based on iron and carbon- of 316l, duplex or super duplex, in order to guarantee the durability of the equipment in a marine environment, from the reverse osmosis unit to the painted container, in order to be protected from this highly corrosive marine environment.
In the case of container deployment, the container must be galvanized to prevent damage to the exterior of the container.
The distribution : of the produced water can be made upstream of reservoirs, network pressure maintenance systems, and downstream for example of metered taps for direct payment of the distributed water, or a network with connections in each household, which pay their bills to the drinking water agency.
Concentrate, or brine : in membrane filtration techniques, this term designates a fluid enriched with substances stopped by the membrane. In the case of desalination, the concentrate is the raw salt water to be treated enriched with salts extracted from the fresh water produced. The discharge is diluted before being released into the sea to minimize the impact on the marine environment.
For brackish water desalination projects far from the coast, the discharge is either evaporated in a specific tank or valorized.
